bnf sodium valproate
Authoritative and practical information on the selection and clinical use of medicines. Update for Valproic Acid in BNF and BNF for Children The following sections in the Valproic acid monograph in BNF Publications will be updated for the next print editiondigital.
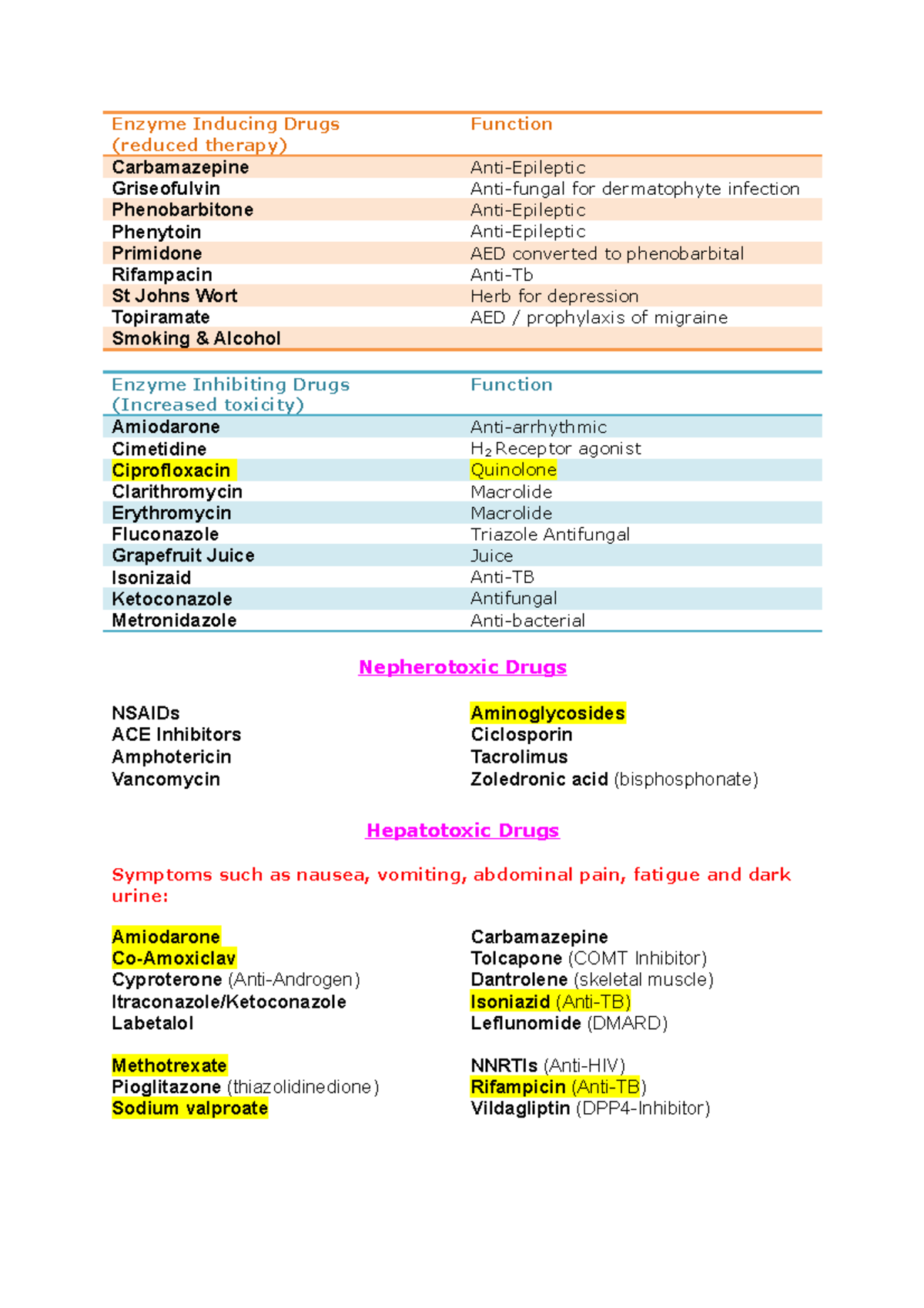 |
| Bnf Drug Summary Enzyme Inducing Drugs Reduced Therapy Function Carbamazepine Anti Epileptic Studocu |
Valproate is highly teratogenic and evidence supports that use in pregnancy leads to neurodevelopmental disorders approx.

. Ada Lovelace Computational Health Lecture. Publishers of the British National Formulary. Hypromellose with dextran 70. Powder and solvent for solution for injection.
Find out more about BNF. The off- lael use of sodium valproate for migraine was first included in the BNF in 2001. Ensure accuracy of documented weight before administration Form 400mg dry powder vial 4mL solvent. Its important to take it as your doctor tells you.
Liquid paraffin with white soft. Sodium Valproate Sodium valproate dosing may be weight based. On Tuesday October 11 2022 at 300 pm. Drug interaction information Severe interactions are highlighted with a red marker.
Sodium valproate has also been used in the UK for migraine prophylaxis. For intravenous infusion dilute with Glucose 5 or Sodium Chloride 09. Valproate is highly teratogenic and evidence supports that use in pregnancy leads to neurodevelopmental disorders approx. See the list of drugs that interact with Valproate.
Dosage The usual dose for treating epilepsy in. Hydroxypropyl guar with polyethylene glycol and propylene glycol. 3040 risk and congenital malformations approx. Muscle pain or stiffness.
Manufacturer advises for intravenous injection give over 35 minutes. Multiple swollen and inflamed skin lesions. Adults and older children aged 12 years and. Valproate semisodium is prescribed as a mood stabiliser in bipolar disorder.
Valproate Interactions Valproate has the following interaction information. Valproate is an anticonvulsant drug used as a mood stabiliser. BNF Code 0408010W0 OpenPrescribing Sodium valproate 0408010W0 Part of chapter 4 Central Nervous System section 48 Antiepileptic drugs paragraph 481 Control. There can be variation in the licensing of different.
Normal menstrual bleeding occurring earlier possibly lasting longer than. For sodium valproate With intravenous use. It is also known by the trade names Epilim and Depakote. It will have been given.
There can be variation in the licensing of different. It is a mixture of two similar ingredients - valproic acid and sodium valproate. Includes information on severity of interaction and the level of evidence for it. 3040 risk and congenital malformations approx.
Sodium valproate is a prescription medicine. Powder and solvent for solution for injection. ET the NLM Intramural Research Program will host Dr. Muscle tension or tightness.
Find out more about BNF interactions information. You can find detailed information about this drug in the official. Noémie Elhadad of Columbia University to.
 |
| Doctors Must Review All Women On Sodium Valproate According To Data Epilepsy Ireland |
 |
| Sodium Valproate The Fetal Valproate Syndrome Tragedy |
 |
| Sodium Valproate Mnemonic For Side Effects Medical Laboratory Science Nursing School Notes Mnemonics |
 |
| Sodium Valproate Medicine To Treat Epilepsy And Bipolar Disorder Nhs |

Post a Comment for "bnf sodium valproate"